What is Thermodynamics? Fundamentals of thermodynamics: System and its types, Surrounding, Heat transfer, Work done
What is Thermodynamics?
Thermodynamics is a branch of physics that deals with the study of energy and its transformations, particularly in relation to heat and temperature. It is concerned with the macroscopic behavior of matter, i.e., the behavior of systems that contain a large number of particles, such as solids, liquids, and gases. The basic principles of thermodynamics govern many phenomena in the natural world, from the behavior of stars to the operation of refrigerators.
Basic terms in thermodynamics:
The fundamental concept in thermodynamics is the “system,” which is a region of space that we are interested in studying. It could be any object, substance, or group of objects or substances that we wish to analyse.
The system is separated from its surroundings by a boundary, which can be real or imaginary. The surroundings include everything outside the system that can exchange energy or matter with the system.
Based on their characteristics, systems can be classified into three types:
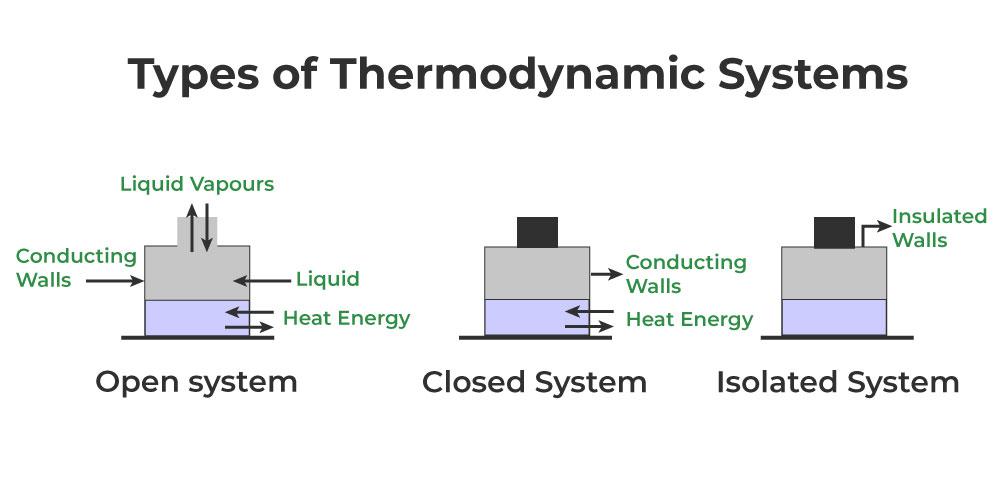
Open system:- A system that can exchange both matter and energy with its surroundings.
Closed system:- A system that can exchange energy but not matter with its surroundings.
Isolated system:- A system that cannot exchange either matter or energy with its surroundings.
The thermodynamic quantities that describe a system and its surroundings include temperature, pressure, volume, and internal energy. Heat transfer is the transfer of energy between a system and its surroundings due to a temperature difference. Work done is the transfer of energy due to the application of a force through a distance.
HEAT
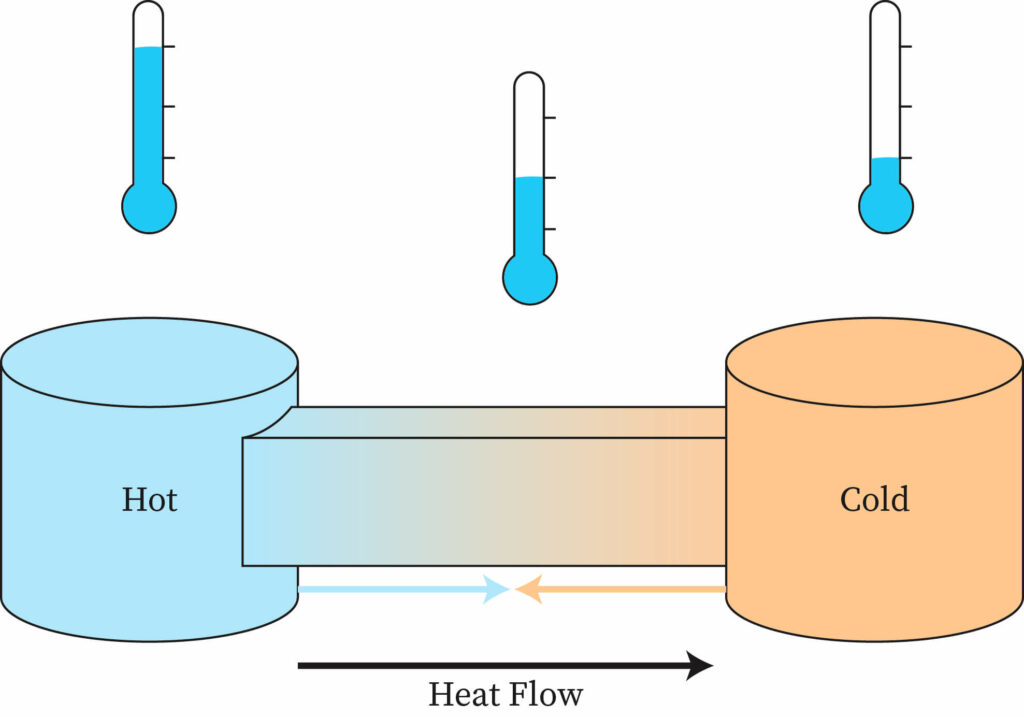
Heat refers to the transfer of energy between objects or systems due to a temperature difference. It is a form of energy transfer that occurs spontaneously from a region of higher temperature to a region of lower temperature.
Q = n c Δt
n=number of molecules
Δt=difference between changes of temp.
c=molar specific heat of gas.
WORK DONE
Work done refers to the measure of energy transferred or expended when a force is applied to move an object through a distance in the direction of the force.

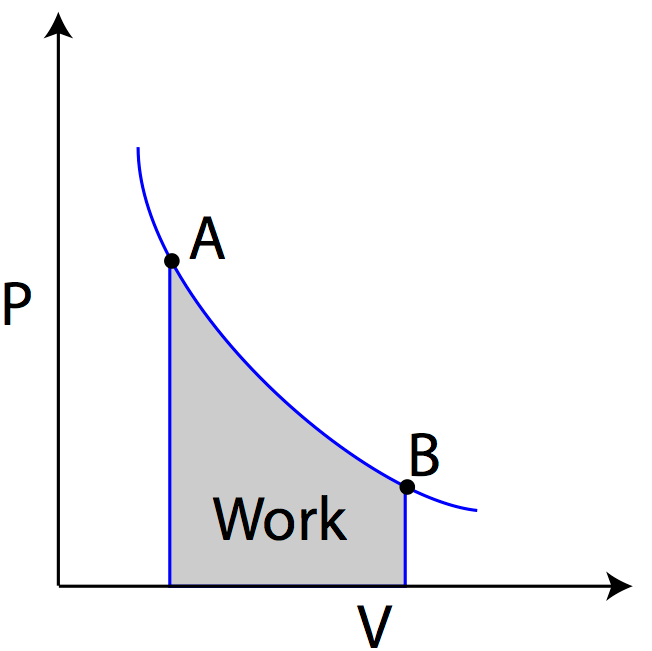
Positive work refers to the work done by the system on its surroundings, indicating that energy is being transferred from the system to the surroundings.
Negative work refers to the work done on the system by its surroundings, suggesting that energy is being transferred from the surroundings to the system.
Note
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.