What is enthalpy?/ what is latent heat of fusion? what is latent heat of vapourisation? / Which is more dangerous for you: 100℃ water or 100℃ steam?What is enthalpy?
Enthalpy is the total amount of heat energy in a thermodynamic system.
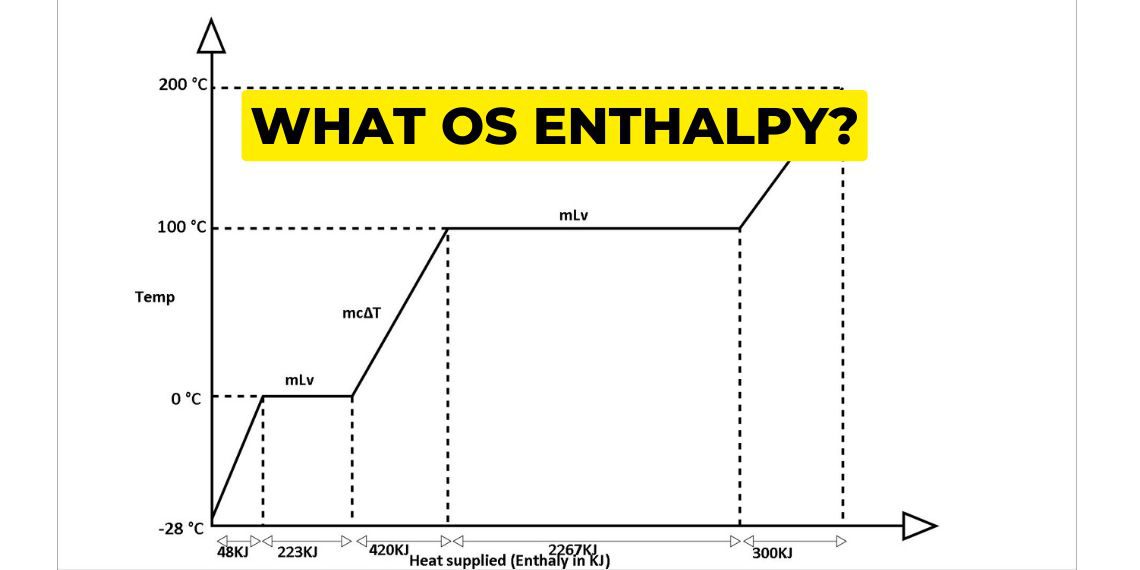
We are understanding this cycle with the help of the temperature enthalpy graph. To understand this concept let 1 kg of ice at -23℃ convert into 200℃ superheated steam.
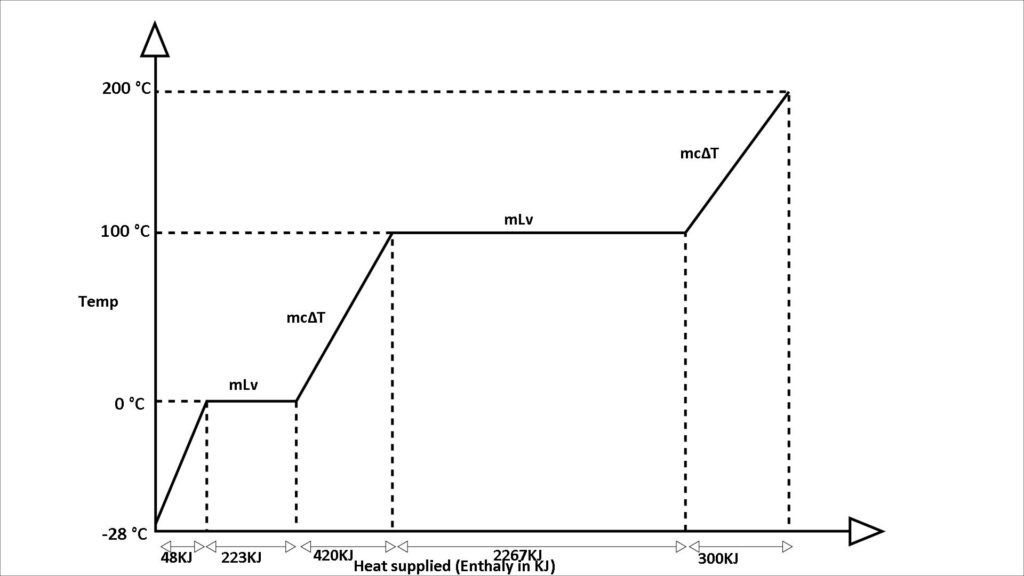
From -23℃ to 0℃ ice gets heated and slowly increases its temperature and it comes in 0℃ ice first. then it converts its state from ice to water.
From -23℃ to 0℃ of 1 kg ice heating process takes near about 48 KJ of heat energy.
Then this again gets heated and 0℃ ice state changes into 0℃ water. Here 333 KJ of heat energy is required to convert into 0℃ water. This is called the latent heat of fusion. On further heating, the 0℃ water and raising its temperature to 100℃ requires 430 KJ of heat energy.
To convert 100℃ 1 kg of water to 100°C steam takes around 2267 KJ of heat. This is called the latent heat of vaporisation.
At 100℃ when all have been converted into steam, now superheating starts from 100℃ to 200℃ steam and takes approximately 300 KJ of heat energy.Heating from 0℃ water to 100℃ water takes 30 minutes time and 430 KJ of heat energy. and 100℃ water to 100℃ steam takes 5 times the heat energy and time. From 100℃ steam to 200℃ superheat steam takes 300 KJ of heat energy and takes less time.
Which is more dangerous for you: 100℃ water or 100℃ steam?

100℃ steam is more dangerous for you because there is more heat energy (Enthalpy) present at this temperature. 100℃ steam has 2267 KJ of heat energy more than 100℃ water. For this reason, 100℃ steam is more dangerous than 100℃ water.
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.